Without them our modern lives would be very different indeed
Powering our phones, laptops, cars and more, batteries are modern technological marvels. Their invention dates back to 1800, when Italian scientist Alessandro Volta first came up with the idea of creating a cell that could generate power.
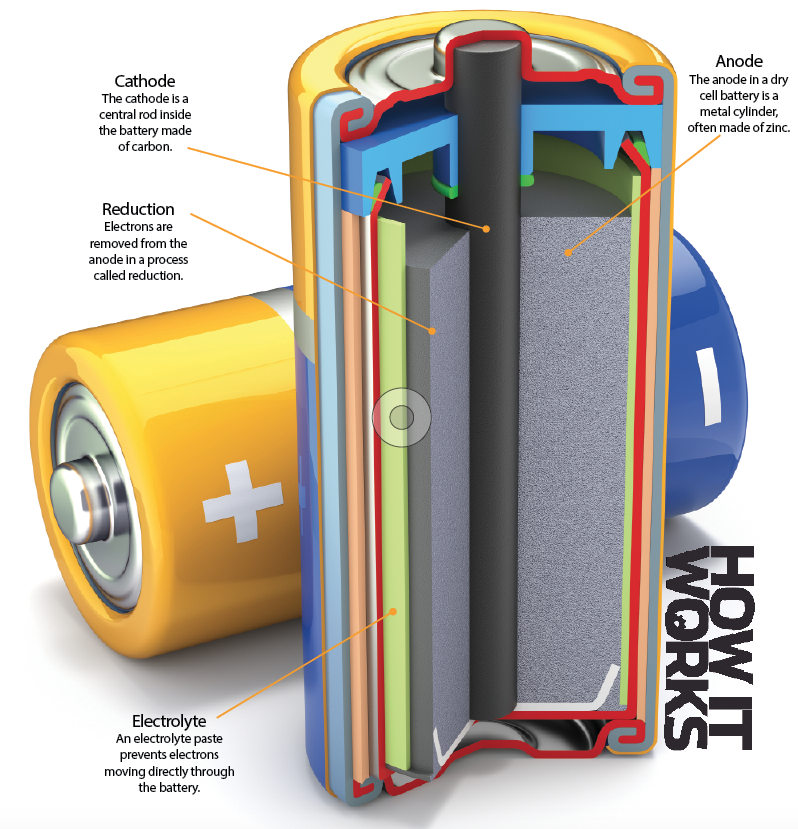
At its heart, a battery involves ferrying electrons between an anode and a cathode. Using an electrolyte – essentially chemical waste – these electrons can’t go through the battery, so instead they go around the outside. As they flow around they complete a circuit, and when plugged into a device this flow of electrons provides power.
Different batteries use different reactions and chemicals, such as zinc and alkaline. At their core, though, they all work in the same way.
Inside a dry cell
The flow of electrons from the anode to the cathode produces electricity