Ever wondered why people with tooth fillings sometimes complain of getting an electric shock like sensation while they nibble on to chocolates from the foil coating or from the silver on desserts? It is not just a fleeting sensation, but is actually a minor electric current tantalizing the nerves underneath the tooth. A momentary cell is formed between the foil and the amalgam of the tooth filling with the acidic saliva acting like the transport medium, the electrolyte.
Fig. 1: An Image Of Battery
History
Electricity has been by far, one of the most important and novel discoveries to mankind. With population getting increasingly mobile, they have gravitated more towards portable solutions of electricity, which manifests itself in the form of Batteries. Electricity has been around us since the beginning of time, but its practical use has been at our disposal since a few hundred years only.
While history stands witness to many artifacts such as the Parthian Battery unearthed in Baghdad, which tell us that the concept had been existent even in early civilizations like Egyptian and Babylonian, their use had been limited to electroplating. In 1660, Otto von Guericke succeeded in generating static charge in the form of sparks on rubbing and turning a sulfur globe. In 1791, Luigi Galvani discovered animal electricity while experimenting on a frog with metallic prongs. Prompted by the findings of this experiment, Alessandro Volta, the inventor of the Electric Battery, initiated a series of experiments using different metals and found out that certain fluids could generate a continuous flow of electricity when used as a conducting medium. This led to the invention of the first voltaic cell commonly known as The Battery in 1800.
Sir Humphry Davy discovered the phenomenon of chemical decomposition (Electrolysis) on passing electricity through substances. In 1802, William Cruickshank designed the first electrical battery for mass production which resembled the flooded battery we still use. In 1859, Gaston Plante invented the first rechargeable battery based on lead acid system which is still very popular and hence came the first secondary cell. In 1899, Waldmar Jungner invented the Nickel-Cadmium battery using Nickel for cathode and Cadmium for anode. It was further improved by many people like Thomas Edison, Shlecht, Ackermann and Georg Nuemann. It remained popular for many years to come until environmentalists became concerned about contamination, if NiCd were disposed off carelessly. This led to the development of Nickel Metal Hydrides and later the popular Lithium Ion batteries. Numerous local, national and international players are involved in this business providing portable battery solutions, a few of the key players being Duracell International Inc., Electric Fuel Battery Corp., Energizer Holdings Inc., GP Batteries International Ltd., Philips, Renata SA, Toshiba Battery Co. Ltd., VARTA Consumer Batteries GmbH & Co. KGaA, Sony Electronics Asia Pacific Pte. Ltd., ZeniPower Battery Co. Ltd., Sanyo Electric Co., LG Chem. Ltd Exide industries Ltd. etc.
What is a Battery
So what actually is a Battery? It is a collection of one or more electrochemical cells in which stored chemical energy is converted into electrical energy. The principles of operation haven’t changed much since the time of Volta. Each cell consists of two half cells connected in series through an electrolytic solution. One half cell houses the Anode to which the positive ions migrate from the Electrolyte and the other houses the Cathode to which the negative ones drift. The two cells are may be connected via a semi permeable membranous structure allowing ions to flow but not the mixing of electrolytes as in the case of most primary cells or in the same solution as in secondary cells.

Fig. 2: Figure Explains Working Of A Typical Battery
Different amounts of voltages are built up according to the separation between the ions in the electrochemical series which results in the flow of ions in the solution and electrons in the external circuitry in the form of current. The performance of the cell continues to dip gradually as the concentration of ions in the solutions decrease, marked by an increase in internal resistance eventually leading to the exhaustion of the battery. The reversibility of this condition classifies the battery into two major categories, Primary and Secondary.
Types of Batteries
1. Primary Batteries: Non rechargeable batteries in which once the electrolyte has been fully used up, energy cannot be readily restored and the battery needs to be discarded. This particular class of batteries has been facing stiff competition from rechargeable battery segment, but still holds a niche market in applications like wrist watches electric keys, toys and military missions. Carbon-Zincor Leclanche batteries and Alkaline-Manganese batteries are the most common primary batteries in consumer applications. Primary batteries offer the highest energy densities, with the Lithium based cells offering more than thrice the energy than corresponding secondary batteries. These do offer an initial cost advantage, but in the long run are more costlier than the secondary batteries owing to their reusability after recharging which is absent in primary batteries.
2. Secondary Batteries: The batteries in which a reversible reaction is responsible for the generation of electricity such that they can be reverted back to the original reactant state fall under the category of secondary batteries. Recharging is effected by passing electric current through the battery.
The oldest form of rechargeable battery is the Lead-Acid battery. Lead Acid battery market is dominating primarily because of the unavailability of any able competitive solution in the market and that they offer lowest cost per watt-hour despite of their low specific energy. The desire to make these batteries maintenance free, the flooded battery type evolved into two variants: Sealed Lead Acid or Gel cells and Valve Regulated Lead Acid (VRLA) Batteries. The flooded battery types are still seen in automobiles, UPS etc. But due to this evolution, the lead acid batteries now cannot charge to their true potential where gassing and water depletion in the acid may take place. Further, these must be stored in fully charged state or else sulfation may cause the degradation of the battery performance. The amount of electric power that can be delivered is often a function of amount of lead present.
Starter Batteries which contain more number of finer lead plates are suited for cranking automobiles with a high surge and fast discharge. Deep cycle batteries which contain more lead are suited for applications requiring longevity and deep discharging as in the case of golf cars and handicap chairs. Absorbent Glass Mat is another improved lead acid battery where electrolyte is absorbed in a mat of glass fibers making the battery spill proof and increasing performance characteristics. The disposal of Lead Acid batteries poses certain environmental problems due to hazards like lead poisoning. Nevertheless it continues to and is expected to retain a large market segment and companies constantly continue to innovate this battery under different names like Firefly Energy, Altraverda Bipolar, Axion Powe etc.
Nickel Cadmium (NiCd) Batteries have a matured technology and are used in places where long service life and economy amidst difficult environmental conditions is required. An easy alternative to this chemistry is the relatively environment friendly Nickel Metal Hydride (NiMH) technology which has improved specific energy ratings. Faster charging and long shelf life coupled with economical pricing and availability in various sizes make them an attractive option for small consumer products. However they seem to suffer from Memory effects hence needing periodic full discharges and also suffer from high self discharge.
Lithium-ion Batteries are the most promising battery systems for portability in consumer products and electric power trains. Lithium is placed on the top in the electrochemical potential list and provides high specific energy per unit weight. It is further divided into three categories, Lithium ion Cobalt, Lithium ion manganese and Lithium ion phosphate which have their own different applications due to varying specific energies, discharge currents and service lives. Other different types of Li-Ion batteries include Li-Polymer and lithium-ion-polymer. Cost reduction, absence of too environment sensitive material and high specific energies have led to the rise of popularity of Li-ion batteries to such an extent that about 34% of the batteries sold are now Li-Ion. These circuits need protection circuits to limit voltage and current and are subject to aging even if kept unused.

Fig. 3: Graph Of Batteries With Theoritical Energy
Another classification of batteries is on the basis of the state of electrolyte viz Wet Cells in which the electrolyte is in liquid state as in Leclanche cells and Dry Cells where the electrolyte is in the form of a gel or paste as in the common zinc-carbon battery. Other types may include ‘Molten salt battery’ which uses a molten salt as electrolyte and ‘Reserve battery’ which are activated only when the internal components are in place.
Charging State & Risk
State-of-Charge
The amount of energy remaining in the battery is often measured by State-of-Charge (SoC). SoC is measured using different techniques ranging from very simple Voltage Method (most inaccurate) to Complex Quantum Magnetism and Impedance spectroscopy. Use of Hydrometer for lead acid battery systems is also common. Laptops and professional portable devices use Coulomb Counting technique to measure SoC. It works on the principle of measuring the current flowing in and out of the battery which would be almost equal when fully charged. Though the performance deteriorates with age of the battery, periodic calibration by full discharge and charging helps keep the system error to around a few percents. Capacity rating is often specified as Ampere-Hourproduct which is indicative of the value of constant current that can be supplied over an hour. For example, a 50mA-Hr battery can deliver 50mA constant current for one hour.
Risks with Batteries
Batteries are prone to accidents like leakage and explosions which are caused mainly due to mishandling or misuse of the batteries. While an explosion may result from misuse like throwing in a flame, attempting recharge of a primary cell, short circuiting, overcharging etc, leakage is mainly either due to manufacturing defects or the storage conditions like temperature, humidity and position. This may result in the leakage of potentially corrosive materials as in the case of a few batteries like Lead Acid and do damage to the equipment in which these have been installed. Use of environmentally dangerous materials like mercury in the batteries have raised widespread concern and have called for various constitutional acts of restrictions on battery materials.
Markets & Future
Market Share of Various Batteries
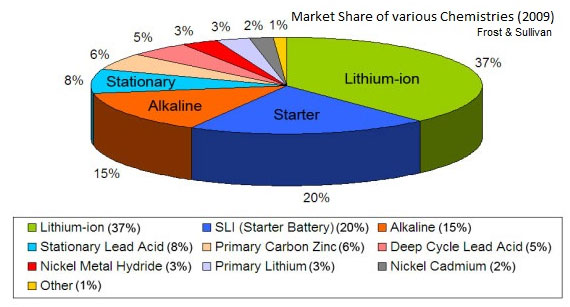
Fig. 4: Figure Showing Market Shares Of Various Batteries
The whole battery market can be broken up into different subsets where different kind of requirements necessitates the use of different battery types. While Lead Acid battery is still the most popular for cranking automobiles, Lithium Ion cells have gained immense popularity among consumer products like laptops, MP3 players and cell phones. Military missions still prefer primary batteries for greater reliability of new primary cells. NiMH and Li-Ion compete in segments of rechargeable and replaceable cells like those in digital cameras and cordless phones with NiMH batteries offering low cost batteries in various formats like different sized (AA, AAA etc) pencil cells. Developing nations seem to be a very potent market for battery sales. Electric cycles and cars being an area of active interest in view of reducing stockpile of fossil fuels invite increased attention to battery technology. Load leveling in wind turbines, solar power and other renewable resources in the form of grid storage, electric power trains etc offer bright future to batteries. The market is continuously growing and is expected to reach $30.5 Billion by 2015.
Future
There are lots of challenges, and each challenge offers an opportunity for this technology to grow. Advancements in the form of super-capacitors and fuel cells are being made. Battery as an ultimate replacement for fossil fuels faces several roadblocks. Powers in terms of Megawatts to provide lift off to an airplane or to drive a ship would be required and the chances that batteries would be surpassing this barrier anywhere in the near future seems bleak. Right now, all that can be said is that if batteries are to replace fossil fuels as a mainstay of human lives, it has very big shoes to fit in.

